


Fundamental ideas of chemistry
What you need to know
Reflections and Exam tips
Atoms, elements and compounds
ATOMS are the smallest building particles that make up matter. Atoms are made up of 3 sub-atomic particles called protons, electrons and neutrons.
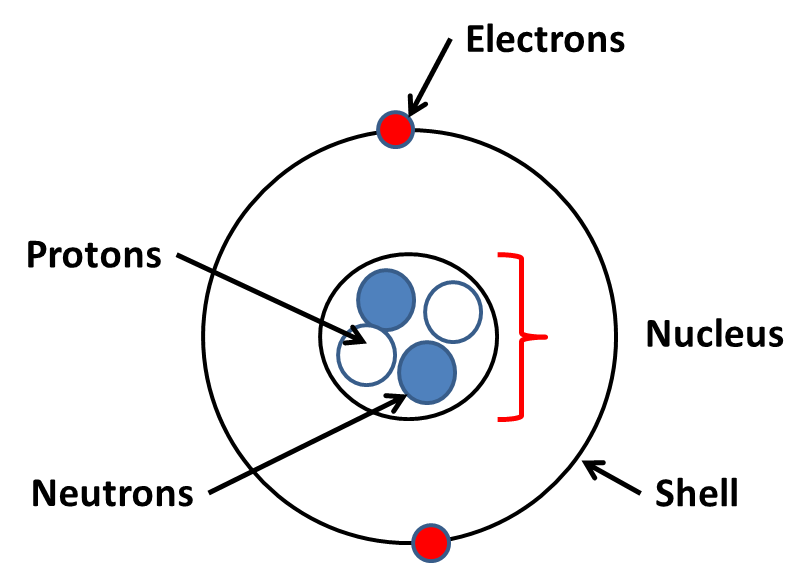
An atoms nucleus contains neutrons and protons. Electrons orbit the nucleus in energy levels (shells).
| Relative Charge | Mass | Location | |
| Proton | + | 1 | Nucleus |
| Electron | - | 1/1840 | Shells |
| Neutron | 0 | 1 | Nucleus |
An atom consist of equal protons (positive) and electrons (negative) making the overal charge neutral. Neutrons have no charge (neutral).
When atoms lose electrons they become positive (+) ions and when they gain electrons they become negative (-) ions. Ions are charged atoms or group of atoms.
The number of protons in the nucleus = atomic number (proton number). Each element has a unique atomic number. Atoms of each element on the Periodic Table is repreented by a chemical symbol for ecample carbon, C.
All substances made up of the same type of atoms are called ELEMENTS. There are about 100 different elements. Elements are shown in the Periodic Table. In general, metals are on the left hand side while non-metals are on the right hand side of the Periodic Table.
Elements react together to produce compounds. Substances made up of two or more different elements that are chemically combined are called COMPOUNDS.
Electronic configuration
Electrons occupy particular energy levels (shells). Electrons occupy from the lowest energy levels (closer to the nucleus).
Ist shell = maximum 2 electrons, 2nd shell = maximum 8 electrons and 3rd shell = maximum 8 electrons.
The Periodic Table is arranged by atomic number with elements in similar groups (columns) having the same number of electrons in their outer shells. The outer shell electrons are responsible for chemical properties. Hence elements with similar electrons in outer shell have similar chemical properties.
The vertical columns are called groups.
The rows are called periods.
Elements with full outer shells are called group 0 (Noble gas). The full outer shell gives a stable electronic arrangement which makes them unreactive.
Chemical reactivity
When elements react they form compounds. The atoms lose, gain or share electrons to form ionic or covalent molecules. Metals and non-metals react to produce ionic compounds through transfer and gaining of electrons. For example, potassium (K) donates its outer shell electron to chlorine to form potassium ion (K+). Chlorine accepts an electron to become a negative ion (Cl-).
Non-metals share electrons to form covalent molecules. For example, two oxygen atoms can share electrons to form an oxygen molecule (O2).
During a chemical reaction mass is conserved as atoms are merely rearranged. No atoms are created nor destroyed according to the law of conservation of mass.
Word equations: sodium + chlorine ——> sodium chloride
Symbol equations: 2Na + Cl2 ——> 2NaCl
Balanced symbol equations must account for all atoms.
You should know the definitions of elements and compounds and the differences between them.
Know the relative charges of electrons, protons and neutrons.
Know how to balance simple chemical equations
Write word equations
Know how to represent the electronic configuration for the first 20 elements